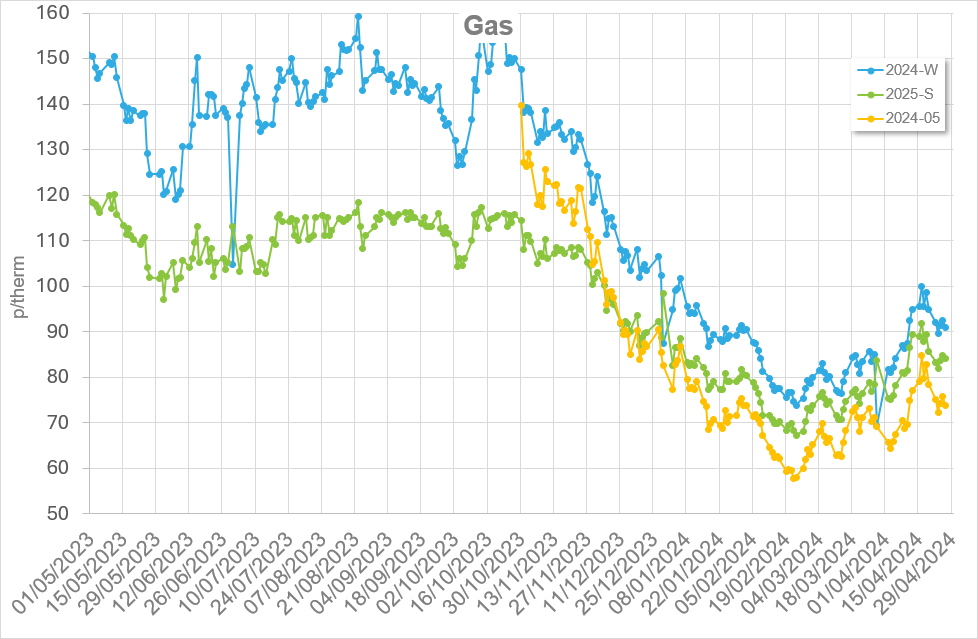
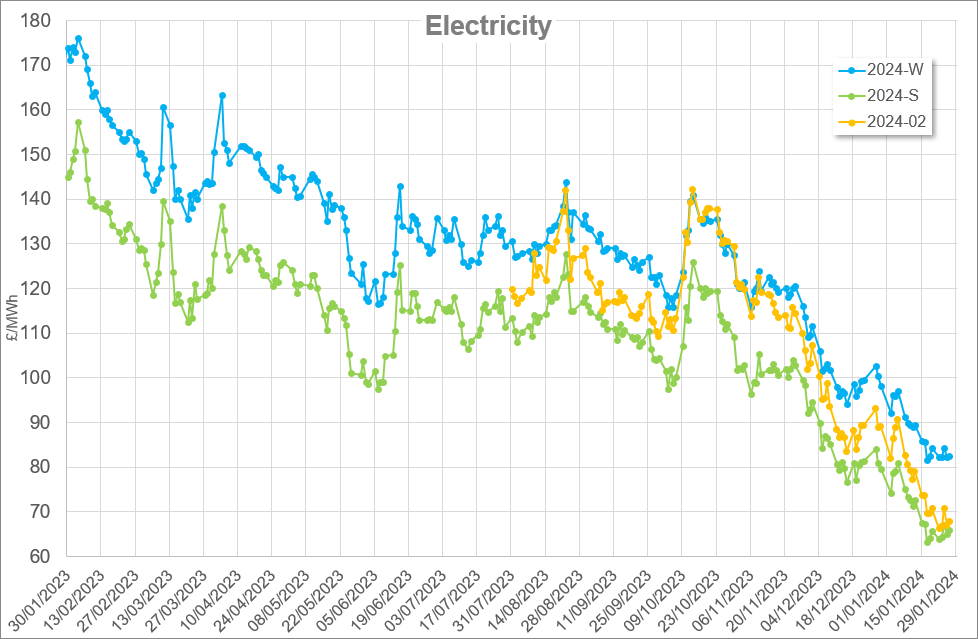
First published May 2018
Thermal storage is the means of storing thermal energy (heat or cooling) so it can be used sometime after it is generated. Find out why you should use thermal storage and what storage medium is best.
Quick links:
What is thermal storage?
Thermal storage is simply a means of storing thermal energy (heat or cooling) so it can be used sometime after it is generated. In most cases in the UK, it is used in heating systems and takes the form of a mass of hot water, ideally approaching 90°C in temperature, and held in some sort of insulated storage tank. The heat for the tank can come from boilers, a combined heat and power (CHP) system or from another heat source such as a heat pump. The stored hot water can be used in a heating circuit to heat a glasshouse.
Why should you use thermal storage?
There are various reasons for using thermal storage. Here we discuss the benefits of storage including more efficient heating systems, smaller heat generators, and heating backup.
To make heat generated now available at a later time
Heat-generating equipment may be used for a number of reasons, which might not always be primarily about a need for heat. For example, this could be the production of CO2 for plant growth, or where an engine is run to generate electricity.
Here, heat is a by-product of the primary process and might be produced when there is no heat demand in the glasshouse. Without storage, a grower may have to exhaust (or dump) this ‘waste’ heat to the atmosphere. With a thermal store, a grower can retain the heat from the daytime CO2 production period and use it during the night when the ambient temperature falls, and the heat demand of the glasshouse increases.
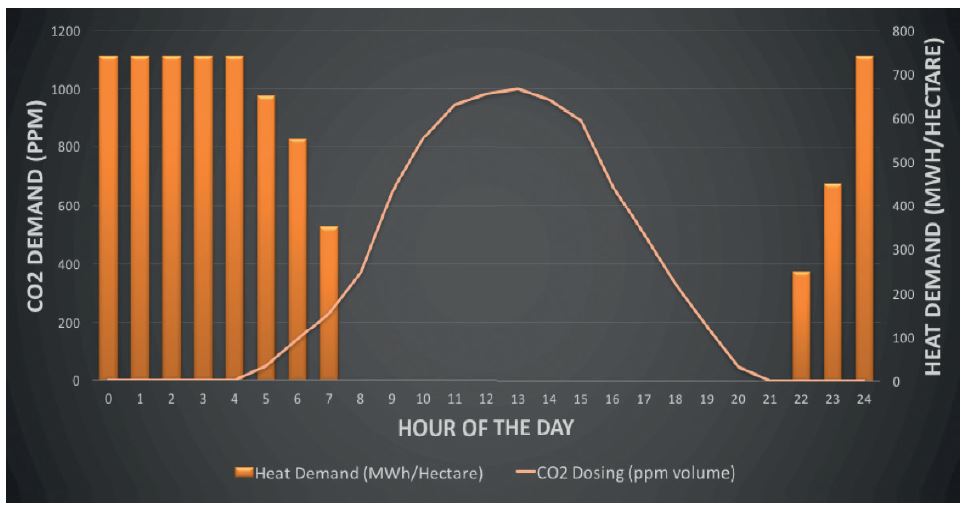
The fuel that would have otherwise been used in a boiler at night can thus be displaced by the stored energy, therefore saving money. Figure 1 shows the daily heat and CO2 demand in a typical salad producing glasshouse and how the daily demand for CO2 and heat occur at opposite ends of the day.
To make systems run more efficiently
The demand for heat in a glasshouse changes seamlessly over time from zero up to a maximum requirement. However, the output of most heat generators, such as boilers, is ‘staged’ in what might be a small number of discrete steps.
Therefore, to meet glasshouse heat demand accurately, a heat generator may have to switch on and off (or ‘cycle’) constantly. In so doing, it operates less efficiently; the constant heating and cooling of the core of the device wastes energy. Using the heat store as a buffer allows the heat generator to operate for longer sustained periods and so avoids rapid cycling and improves efficiency.
It is the heat delivery from the store that copes with the seamless changing demand from the glasshouse, not the heat generator.
With the introduction of heat sources like biomass boilers, the ability to buffer heat output is especially important, as this type of boiler does not lend itself to even infrequent cycling. A heat store can also act as a safety mechanism; when heat demand stops, but the boiler is still hot, the heat store will continue to absorb heat from the boiler, preventing it from overheating.
To make heat generators smaller
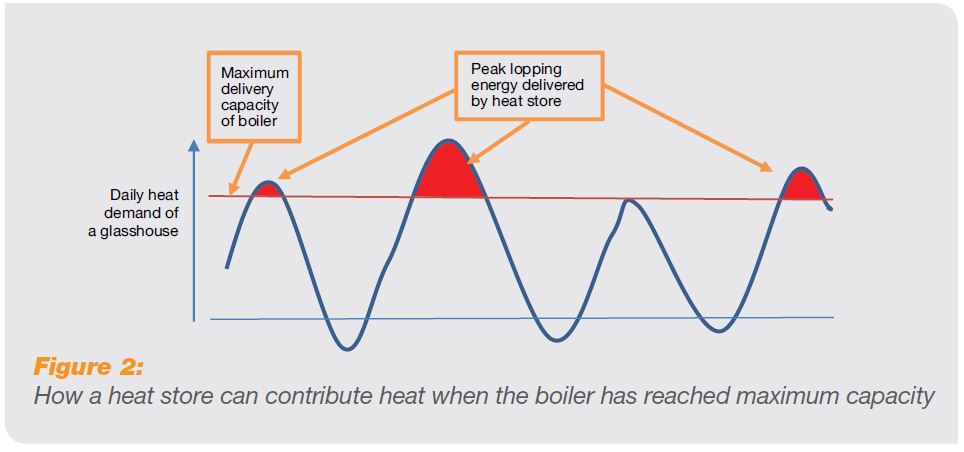
A heat generator which has no heat store has to be sized to meet the peak heating demand of the glasshouse. With a heat store, however, it is possible to use the store to ‘peak-lop’ heating demand and so size the boiler according to sustained demand (Figure 2).
To allow multiple heat generators to co-ordinate effectively
A well-operated heat store can be regarded as the primary source of heat for a glasshouse, with each heat generator used to replenish the hot water in the store rather than to heat the glasshouse directly. If configured in this way, each heat generator can be operated at its most efficient point and most appropriate time without reference to the heat demand of the glasshouse. For instance, CHP can be operated when electricity or CO2 is required, or biomass boilers fired over long periods even during periods of cyclic heat demand without compromising the efficient operation.
To give heating backup
No one should install a heat store for backup purposes only, but it can be useful if things go wrong. Heat stores can be invaluable in meeting short-term heat demand when primary heating generators fail. They also allow the possibility of introducing alternative heat sources in a seamless way. For example, a new boiler or heating device need only be connected to the heat store leaving the primary heating circuits feeding the glasshouse undisturbed. The store forms an interface, or buffer between the heat producing equipment and the glasshouse heating system.
What is the best medium to store heat?
Although water is often the best medium to store heat, we also take a look at geological storage and phase change materials.
Water; probably the best medium
Is water the best medium for heat storage? The answer is, probably, yes. Water is:
- Cheap
- Safe
- Readily available
- High in thermal capacity
- Very adaptable – in terms of its use through heating systems and heat generators
All of these characteristics make it an ideal heat storage medium especially in a purpose-built, above ground store. There are other potential options for heat storage including geological storage or in ‘phase change’ material.
Geological storage
In some parts of the world, geological conditions lend themselves to heat storage underground in rock or in water aquifers. UK conditions are not generally favourable to this method of storage, although there are some configurations associated with heat pump applications which can use underground heat storage to some advantage. Where the geology of underground rocks allows storage of water in discrete areas without mixing, it is possible to have a ‘warm water area’ and a ‘cool water area’. In summer a heat-pump can be used to store heat in the warm water area and use it to heat during the winter. The cycle can be reversed for summer cooling.
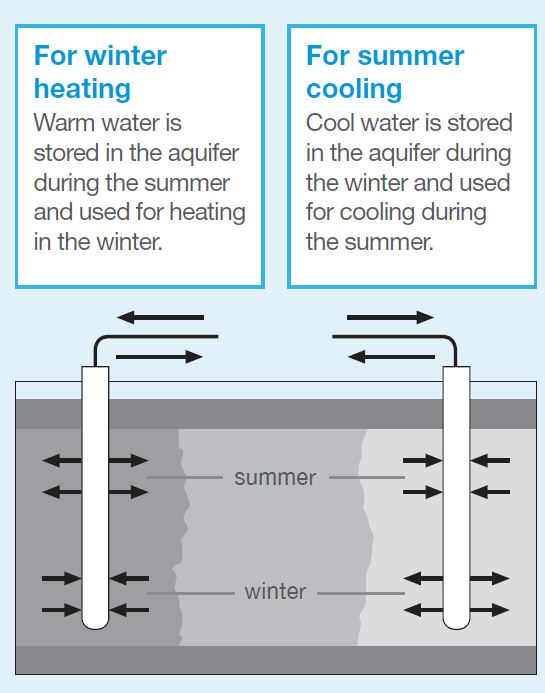
For winter heating, warm water is stored in the aquifer during the summer and used for heating in the winter.
For summer cooling, cool water is stored in the aquifer during the winter and used for cooling during the summer.
Phase change materials
There have been suggestions that the application of ‘phase-change’ materials – which store heat energy at a constant temperature as ‘latent heat’ through a phase-change between solid and liquid state – might bring benefits, such as a reduction in volume of material needed to store a given amount of energy and an ability to store more energy at a lower temperature.
Water takes up a massive amount of storage volume, as all heat stored in horticultural water systems is done in the form of ‘sensible’ heat (i.e. heat absorbed or given up during a change in temperature of the stored water).
In a phase change (a change between solid and liquid or liquid and gas), energy absorbed/emitted per unit volume is much greater than with a conventional system, which requires a change in temperature for energy storage. Hence storage vessel size can be reduced and more stable temperatures in the storage medium can be maintained.
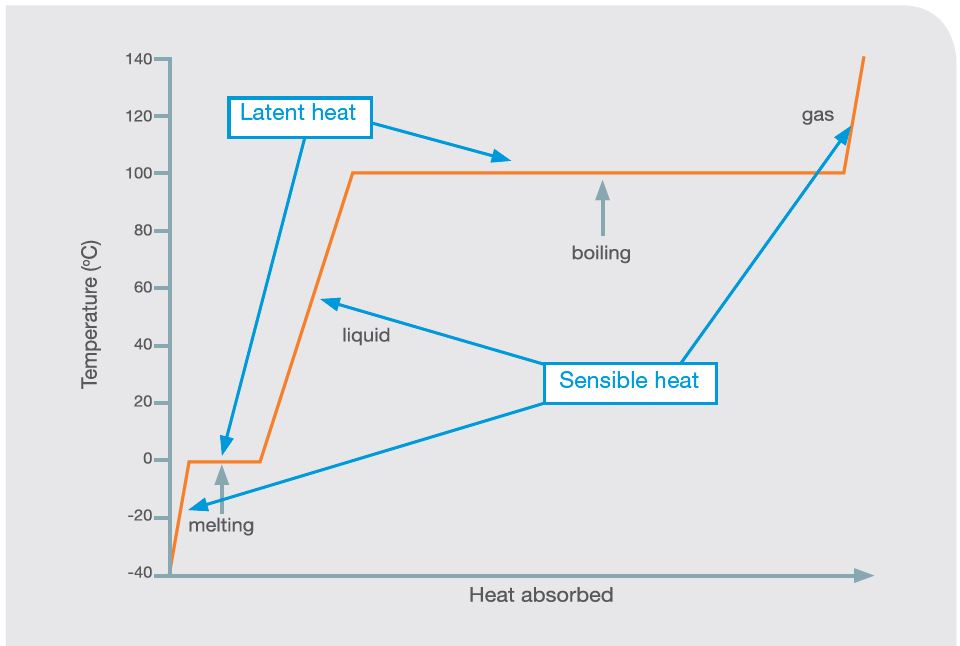
Temperatures at which changes of state take place vary between materials. With water at normal atmospheric pressure, the solid-liquid transition takes place at 0°C (freezing point) and liquid-gas transition at 100°C (boiling point).
Figure 4 shows the relationship between heat absorbed and temperature, and the effect of state change for water. Other materials have different temperatures at which they solidify (freeze) or evaporate (boil). Choosing an appropriate transition temperature is important in making a system work well.
In practice, the most useful change of state is that which takes place between the solid and liquid phase. The change from liquid to gas tends to introduce problems from large changes in material volume and the containment of gases at higher than atmospheric pressure.
Commercially, phase change systems have not found favour, as the benefits have not been enough to justify their higher capital costs. Simply speaking, water storage is cheap and the savings in volume reductions offered by phase change materials have simply not been enough to warrant their high cost.
What does the future hold?
Looking to the future, however, they may come into their own when used with heat pumps. Since the temperature output of heat pumps is lower than a boiler, much more storage volume is required for them to store the equivalent amount of heat. In fact, in many cases, water-based heat storage would not be economical with a heat pump. A phase change material operating at about the temperature output of a heat pump could solve this by dramatically reducing required storage volumes. Only time will tell if there is enough benefit in this area to make phase change materials a real commercially viable option in this area.
An explanation of Latent heat and Sensible heat
Latent heat is the energy related to changes in state between liquids, gases, and solids when there is no change in temperature of the material, just a change in state, i.e. when water boils and turns to steam.
Sensible heat is the energy related to changes in temperature of a gas or object, where there is no change in state, i.e. water being heated from 10 – 95°C.